Mechanisms of Presynaptic Homeostatic Plasticity
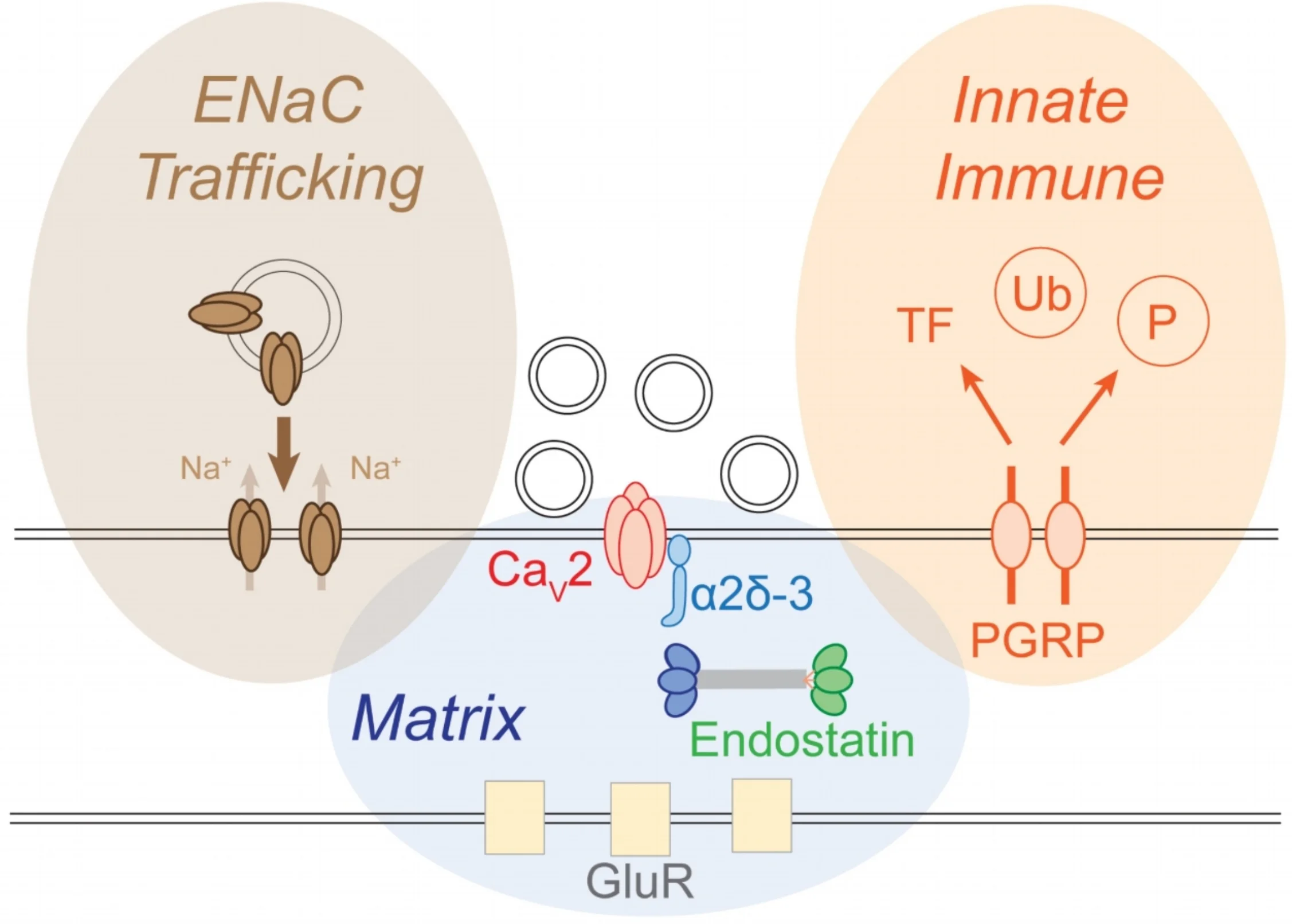
We used the powerful forward genetics of Drosophila to screen genes that, when mutated, block presynaptic homeostatic plasticity. All of our screens have been based on direct, electrophysiological measurement of synaptic transmission, entailing more than 12,000 intracellular recordings and, recently, coverage of the nearly 50% of the entire Drosophila genome. We are converging upon a set of essential signaling systems, diagrammed below. Many of the molecular mechanisms that we have identified are completely novel within the nervous system, highlighting the importance of unbiased gene screening as a path to discovery. Recent mechanisms include: 1) ENaC channel trafficking to the presynaptic membrane (Younger et al., 2013), 2) a novel intercellular signal achieved by Endostatin (Wang et al., 2014) and 3) the action of an innate immune receptor never before studied in the nervous system of any organism (Harris et al., 2015). Future efforts will include translation to mammalian systems and models of neurological and psychiatric disease. In addition, we will pursue systems biology approaches to understand how recently identified signaling systems are integrated to achieve the coherent, robust homeostatic control of synaptic transmission.
Diagram of a synapse. The presynaptic release site is centered upon the presynaptic calcium channel (Cav2). Neurotransmitter vesicles are released opposite to postsynaptic glutamate receptors (GluR). We have defined three major signaling systems that converge to achieve the homeostatic control of synaptic vesicle release, each defined by a brown, blue or orange oval. These mechanisms include the regulated insertion of ENaC channels (brown), release of signaling factors from the extracellular matrix (blue) and activation of innate immune signaling (orange).