The Stable Brain
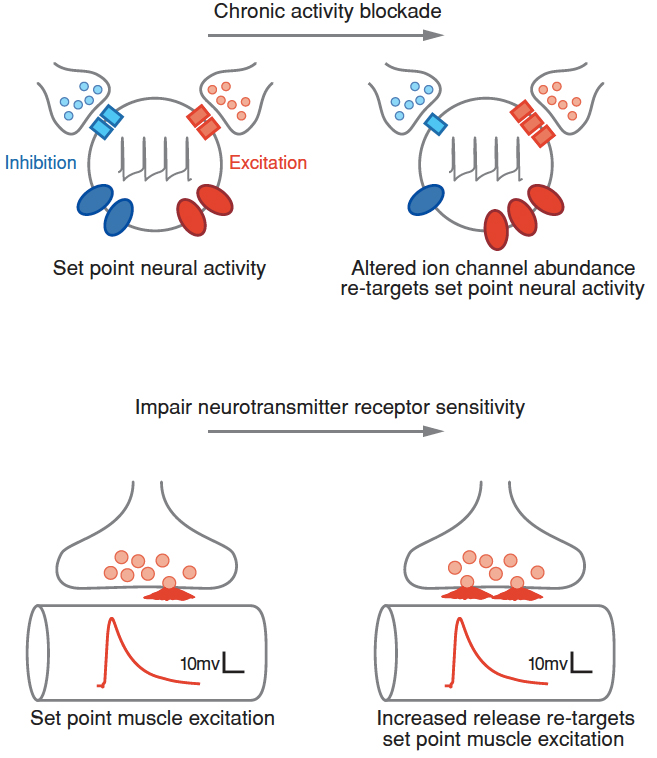
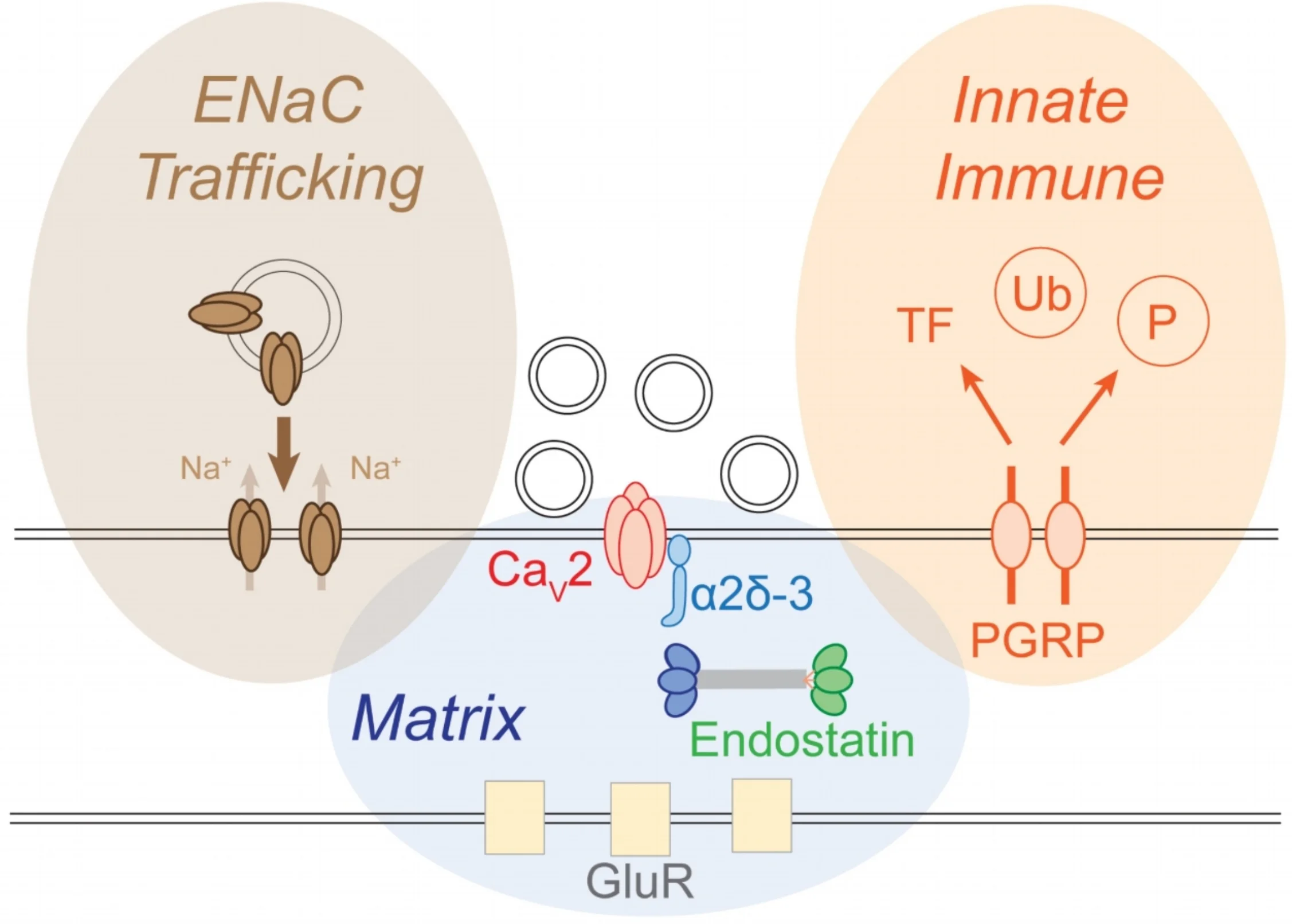
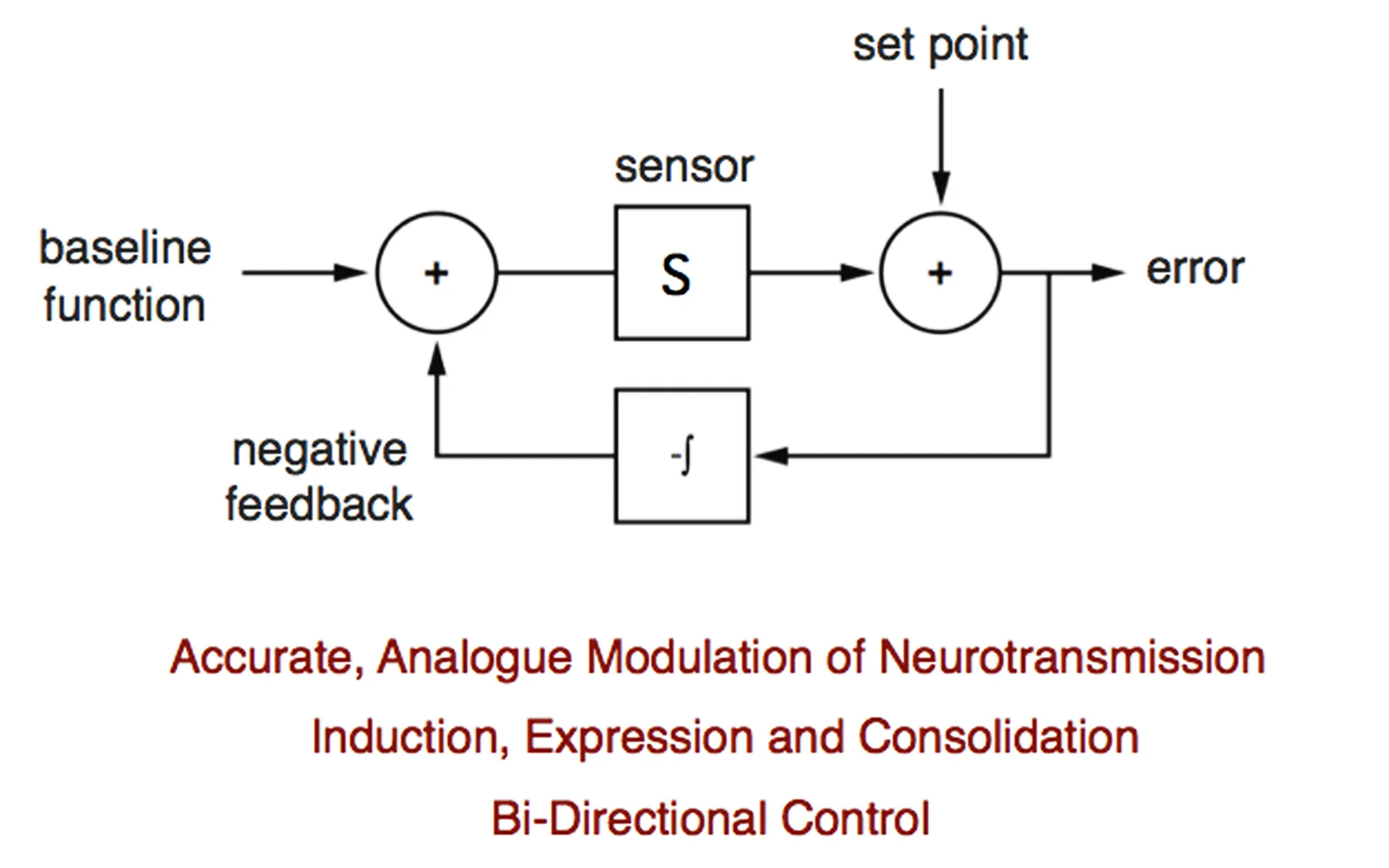
The brain is astonishing in its complexity and capacity for change. This has fascinated scientists for more than a century. But, a paradigm shift is underway. The plasticity that drives our ability to learn and remember can only be meaningful in the context of otherwise stable, reproducible, and predictable baseline neural function. Without the existence of potent mechanisms that stabilize neural function, our capacity to learn and remember would be lost in the chaos of daily experiential change. This underscores two great mysteries in neuroscience:
1. How are the functional properties of individual nerve cells and neural circuits stably maintained throughout life?
2. In the face of potent stabilizing mechanisms, how can neural circuitry be modified during neural development, learning and memory?
We are seeking cellular and molecular answers to these fundamental questions. Our progress promises to open new avenues for the treatment of neurological diseases that are characterized by neuronal malfunction including epilepsy, autism, post-traumatic stress disorder and psychosis.
OPPORTUNITY
We are actively seeking applications for postdoctoral fellows to join our research team. If interested, please contact Graeme Davis directly, contact information on this site.